
SOLVED: Write the net-ionic equation for the reaction between calcium carbonate and aqueous hydrochloric acid. First; write out each species as it exists in solution. Which of the reactants is (are) water-soluble?

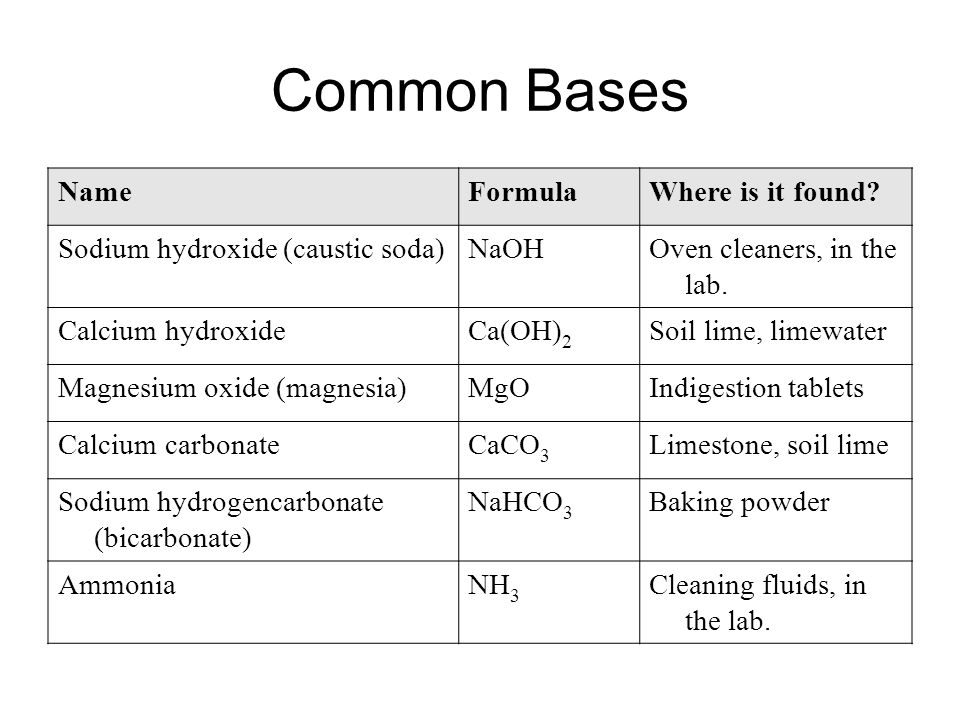
Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download

Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
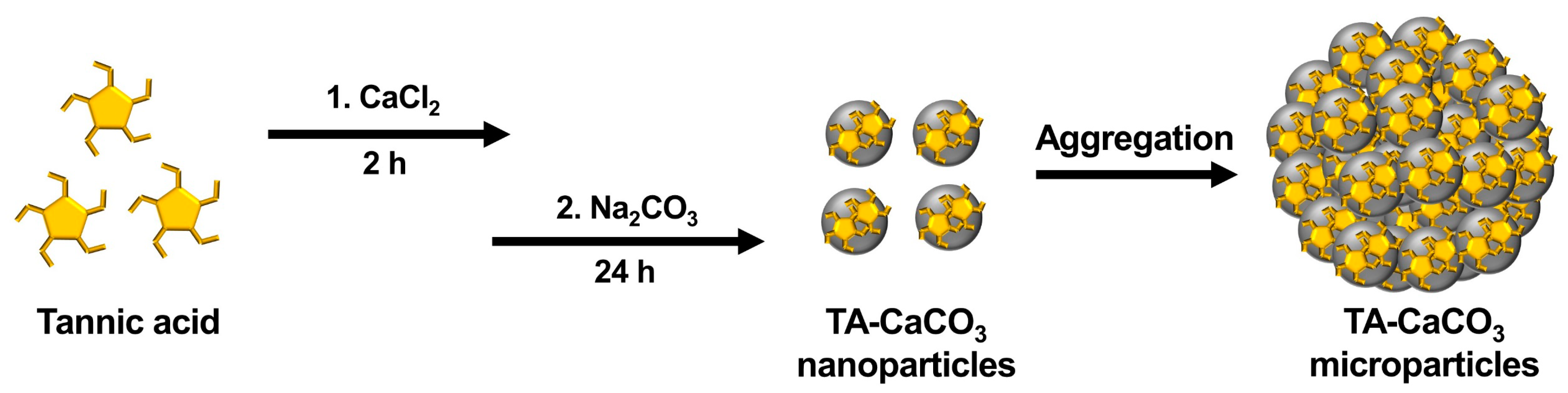
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects






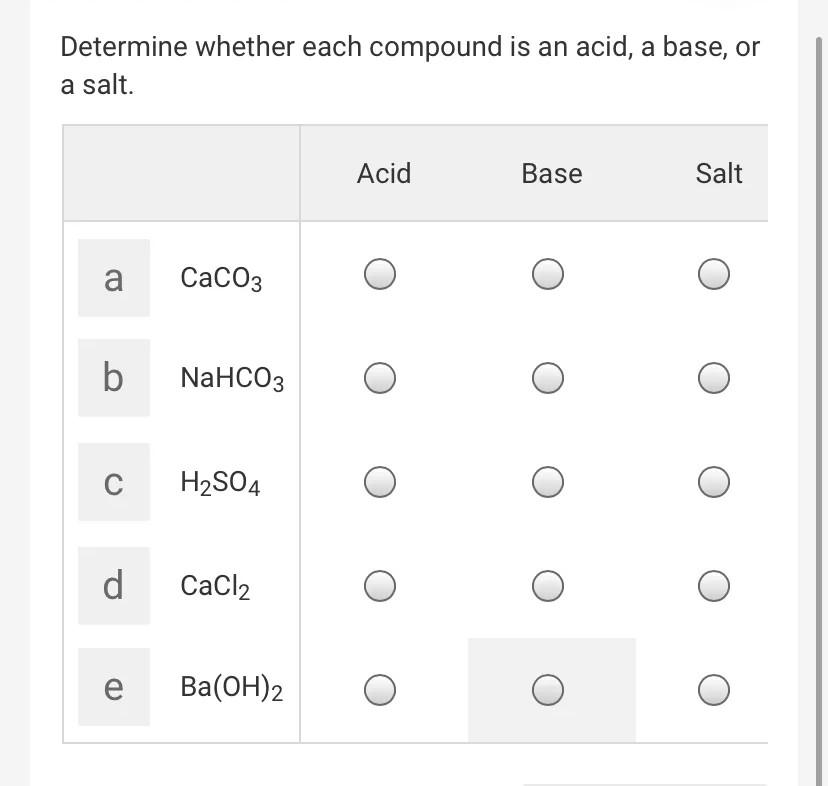

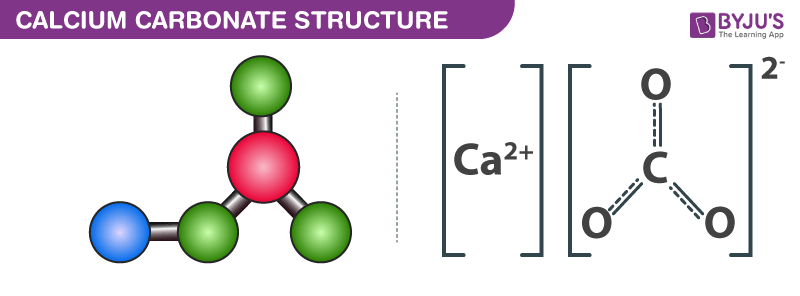
![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)

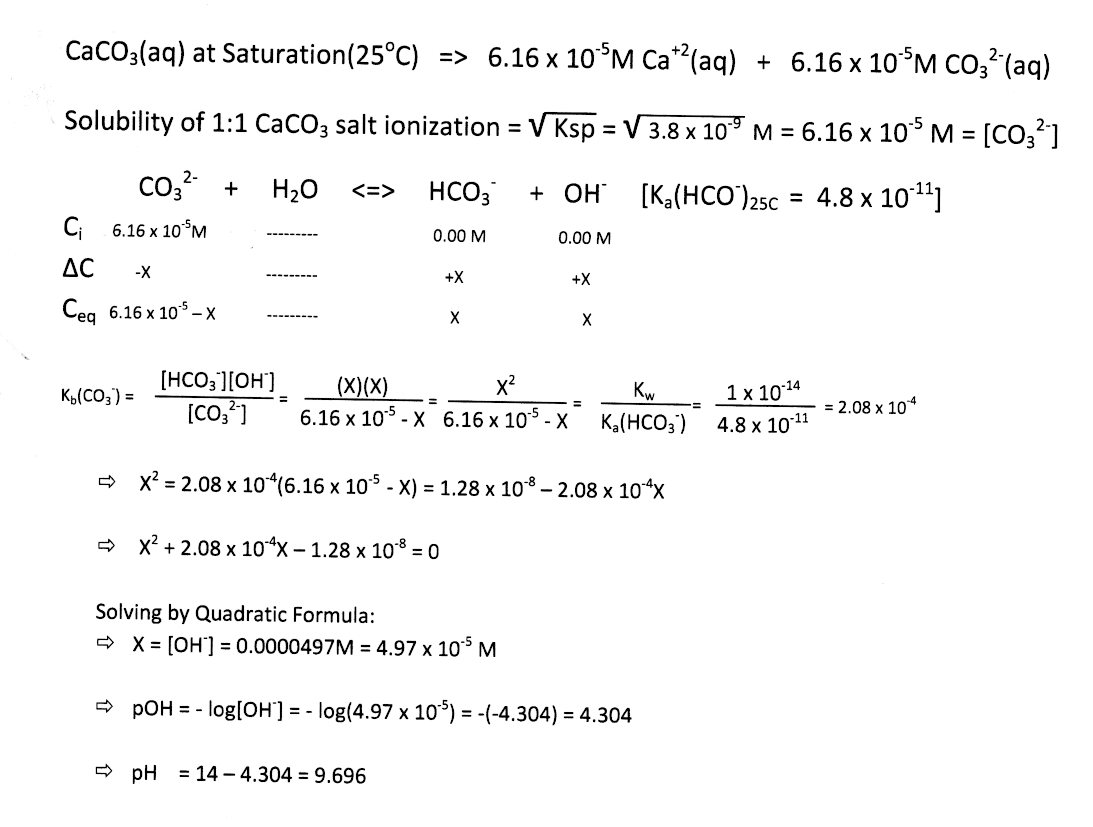

![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/475685cc-4d58-4e74-a739-8cbc5d1c10f8/q8---parent-acid-and-base-of-calcium-carbonate---teachoo.jpg)


