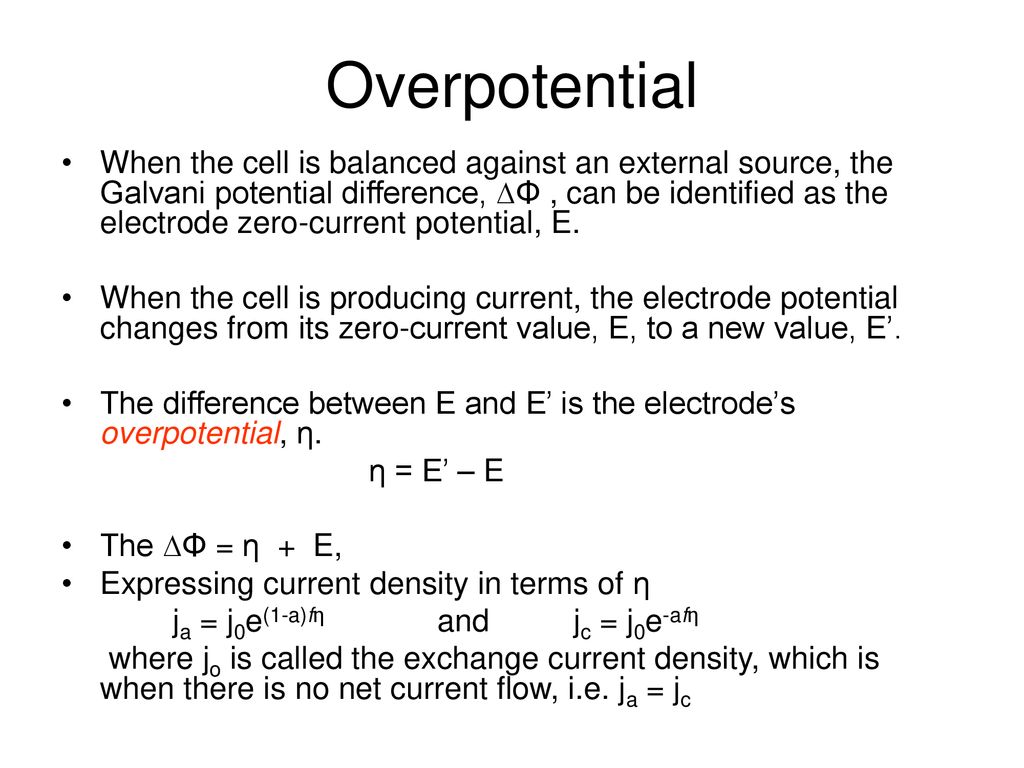
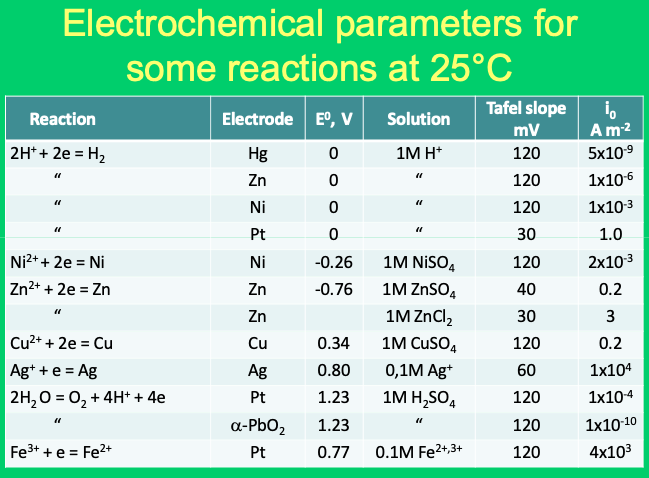
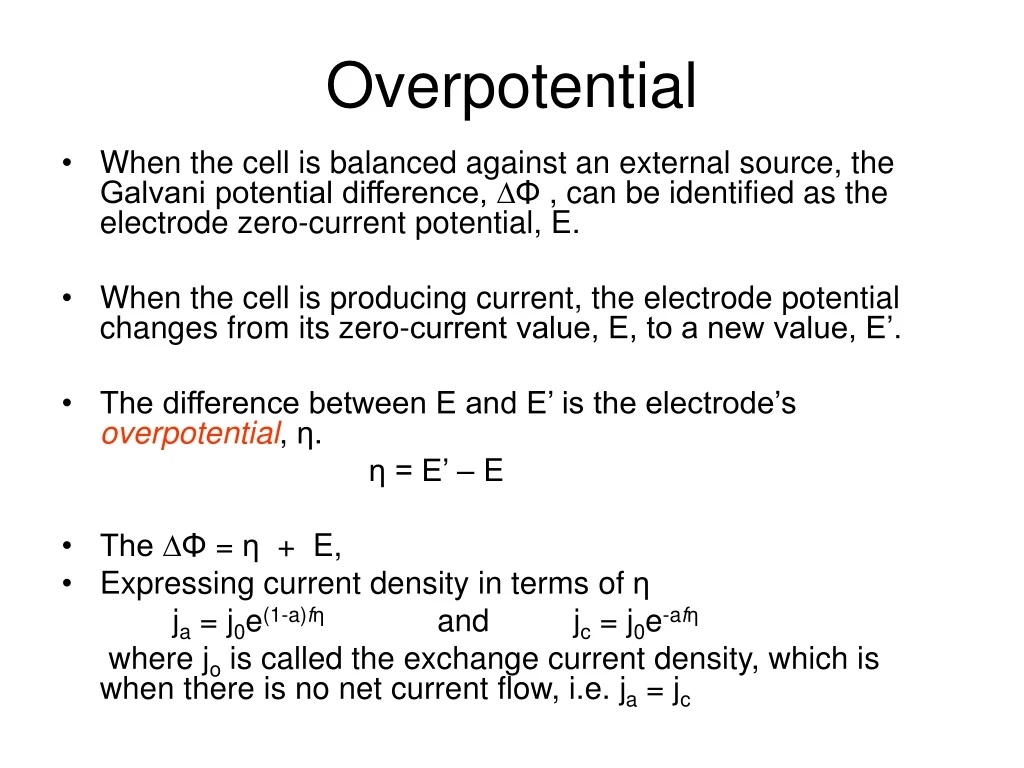
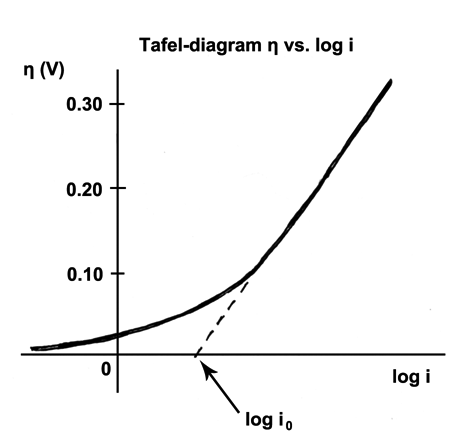
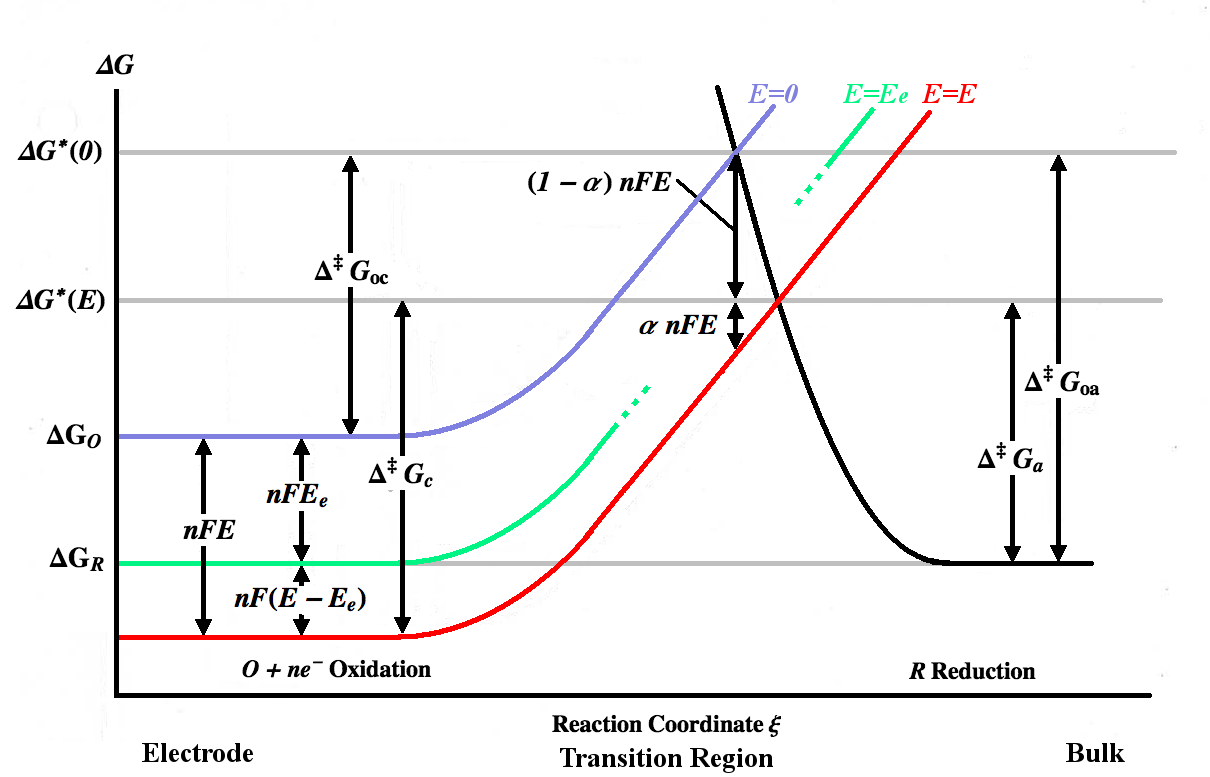
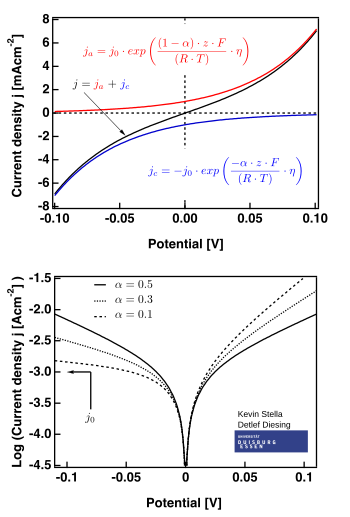
How to calculate exchange current density from Tafel plot for 2 chamber MFC? How other parameters like act potential/no of electrons are determined? | ResearchGate

Model‐Based Overpotential Deconvolution, Partial Impedance Spectroscopy, and Sensitivity Analysis of a Lithium‐Ion Cell with Blend Cathode - Quarti - 2021 - Energy Technology - Wiley Online Library
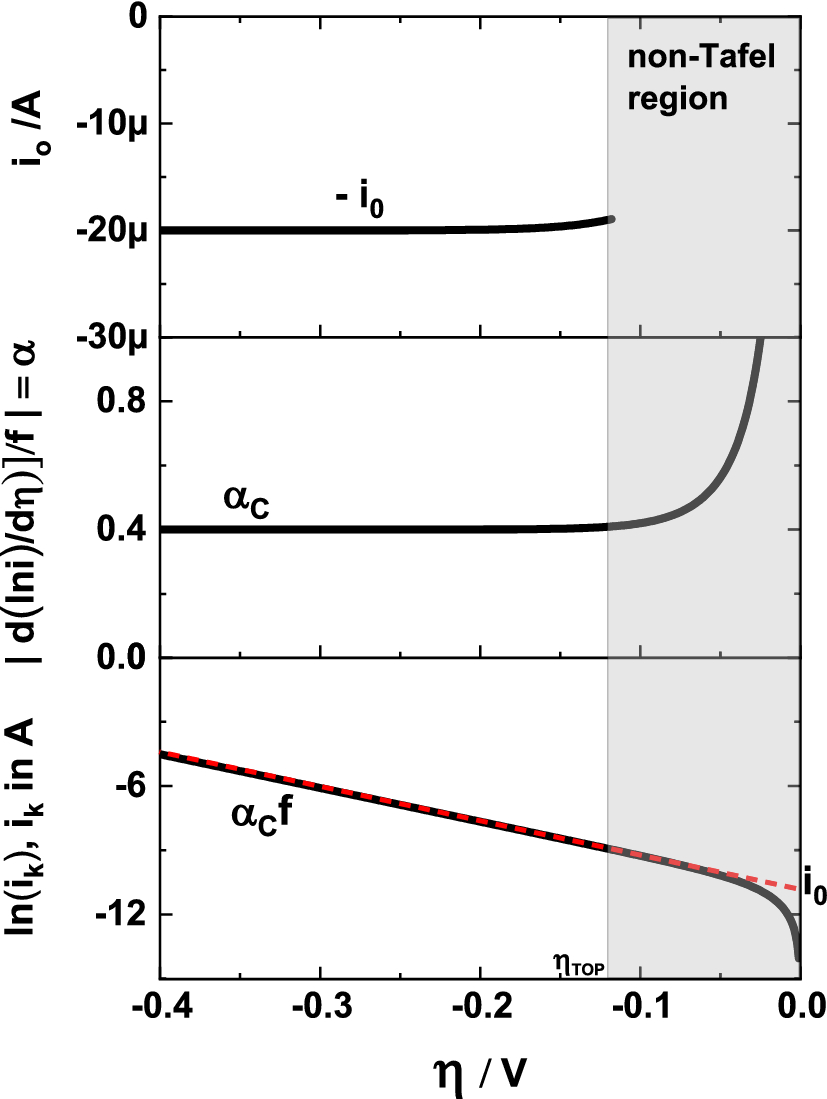
A simple and effective method for the accurate extraction of kinetic parameters using differential Tafel plots | Scientific Reports
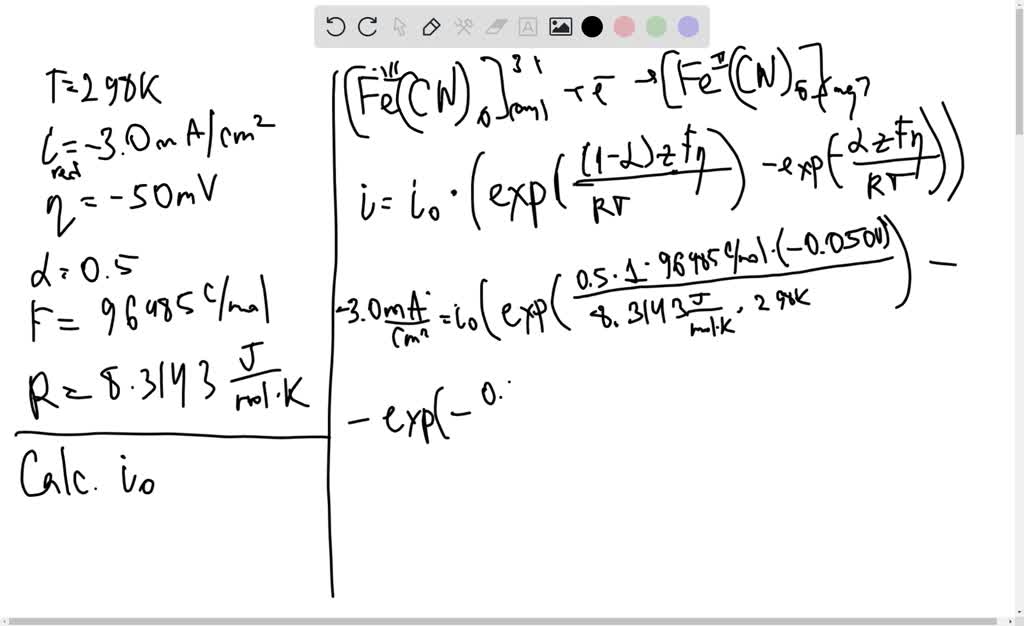
SOLVED: For a reaction at 298 K in which n = 1, α = 0.5, i0 = 2.0x10-6 Acm-2, and mass transfer effects can be ignored, calculate the cathodic current density flowing
Buffer pKa and Transport Govern the Concentration Overpotential in Electrochemical Oxygen Reduction at Neutral pH

pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Transport in Electrode Reaction Kinetics | CCS Chemistry